Leukemia/Lymphoma CAR-T Therapy
Director Tong Chunrong's Team:
CD19 CAR-T Therapy for R/R B-ALL
The team has employed CD19 CAR-T therapy for patients with relapsed/refractory acute B lymphoblastic leukemia(R/R B-ALL),achieving a complete remission(CR)rate of over 90%.
CD22 CAR-T Therapy for Adult R/R B-ALL
The team administered CD22 CAR-T cell therapy to adult patients with relapsed/refractory acute B lymphoblastic leukemia(R/R B-ALL),with 78.9%achieving complete remission with incomplete blood count recovery(CRi).Furthermore,following CD22 CAR-T cell therapy,bridging to transplant treatment can enhance the CR rate in patients.
Director Zhang Yonghong's Team:
Exploration of CAR-T Therapy in R/R MBL
Previously,the team made a breakthrough innovation by using sequential infusion of CD19/CD22/CD20 CAR-T cells to treat refractory/relapsed Burkitt lymphoma(R/R BL),achieving an 18-month progression-free survival(PFS)rate of 78%.These results have been published in BLOOD and Blood Advances.

The team,building on the classical treatment protocol for children with mature B-cell lymphoma(MBL),the LMB89/96 protocol,modified the CNCL-B-NHL-2017 protocol,achieving a 2-year overall survival(OS)rate of 93.4%and a 2-year event-free survival(EFS)rate of 91.3%.To address the challenges of progression and relapse in MBL,the team also conducted the largest domestic multicenter clinical study on children with R/R MBL,the results of which show
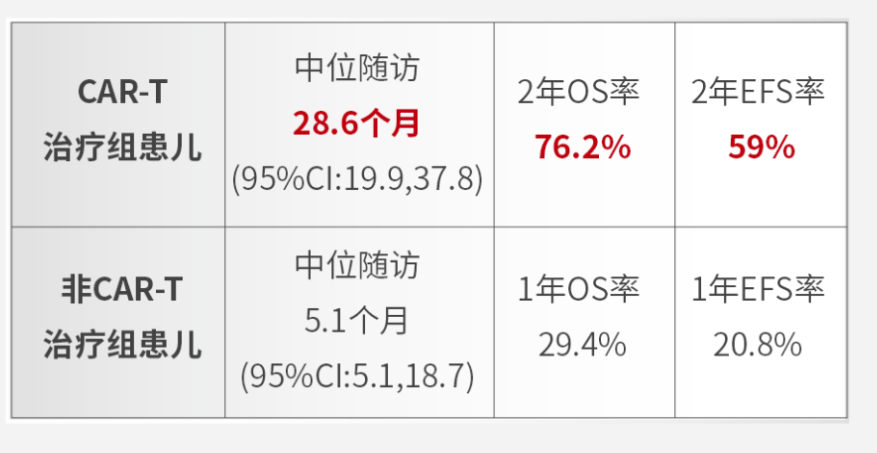
The efficacy of the CAR-T treatment group was significantly higher than that of the non-CAR-T group(including second-line chemotherapy,targeted drug therapy,or monoclonal antibody therapy),confirming the excellent efficacy of sequential CAR-T treatment for R/R MBL.These results were presented as an oral report at the 17th International Conference on Malignant Lymphoma(ICML2023).
Prognostic Assessment of CAR-T Treatment for R/R MB-NHL
In 2023,the team was the first internationally to report the occurrence frequency of ARID1A in mature B-cell non-Hodgkin lymphoma(MB-NHL),as well as its impact on CAR-T treatment.The study found that children with MB-NHL carrying mutations in ARID1A or co-mutations in TP53 and ARID1A were insensitive to initial standard chemotherapy and subsequent CAR-T treatment,and this was associated with disease progression or relapse.This suggests that testing for ARID1A and TP53 mutation status has prognostic value and may predict poor treatment outcomes with CAR-T treatment for R/R MB-NHL.These results have been published in Cancer Cell International this year.Supported by these findings,clinical considerations for children with these mutations should include other treatment modalities,such as chemotherapy combined with targeted therapy or transplantation,and the active exploration of new treatment options is warranted.
The team has focused on exploring the driving gene mutations in Chinese children with lymphoma and their impact on clinical treatment,presenting for the first time to the world the molecular characteristics of Chinese children with MB-NHL and T-cell lymphoblastic lymphoma(T-LBL).The research findings indicate:TP53 mutations are more common in Chinese children with R/R MB-NHL than in non-R/R MB-NHL children and are associated with poor prognosis;the presence of NOTCH1 or FBXW7 mutations in T-LBL suggests a good prognosis.These research results will help clinicians to"tailor"treatment plans for children carrying these high-risk genetic variants.
Director Pan Jing's Team:
Autologous CD7 CAR-T Treatment for R/R B-ALL/LBL
Utilizing autologous CD7 CAR-T therapy for refractory/relapsed acute T lymphoblastic leukemia/lymphoma(R/R T-ALL/LBL),an 85%MRD-negative complete remission(CR)rate was observed at one month post CAR-T infusion.During a median follow-up period of 9.2 months(range 3.3-24 months),among the 13 patients who underwent transplantation,9 achieved MRD-negative CR.No instances of GVHD or infection events were observed in any of the patients.
Donor-Derived CD7 CAR-T Treatment for R/R T-ALL/LBL
After a median follow-up period of 27.0 months(24.0-29.3 months),the objective response rate(ORR)and CR rate were 95%(19/20 patients)and 85%(17/20 patients)respectively,with a 24-month progression-free survival(PFS)and overall survival(OS)rates of 36.8%and 42.3%.The median PFS and OS were 11.0 months and 18.3 months,respectively.The results suggest that donor-derived CD7 CAR-T cell therapy could be a viable salvage therapy for R/R T-ALL,offering patients long-term survival benefits with manageable safety.
Donor-Derived CD5 CAR-T Treatment for R/R T-ALL/LBL
The study included 14 R/R T-ALL patients,of which 10 exhibited low expression/loss of expression of CD7(n=3)or low expression after CD7 CAR-T treatment(n=7).Twelve patients achieved complete remission by day 30 and were followed up for a median duration of 10.9 months(range 1.3 months-16.7 months).
Request an Appointment
Whether you are ready to make an appointment now or have questions for our expert team, we are standing by to help.
Email: international.service@gobroadhealthcare.com Phone: +86 010-83605002/010-83605200 Online Access
















